25+ Buffer Capacity Calculation
Web A buffer solution more precisely pH buffer or hydrogen ion buffer is an acid or a base aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice. To measure the buffering.

Comprehensive Middle Down Mass Spectrometry Characterization Of An Antibody Drug Conjugate By Combined Ion Activation Methods Analytical Chemistry
Web Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture.

. Web Buffer solution pH calculations Google Classroom About Transcript Example of calculating the pH of a buffer solution using the Henderson-Hasselbalch equation including the pH. Web The buffer capacity is defined as the amount of acid or base you can add without changing the pH by more than 1 pH unit. Calculate the pH after 10 mL of 010 M NaOH is added.
Chem 1969 18 427-436 DOI which states. Web Calculate the pH of an acetate buffer that is a mixture with 010 M acetic acid and 010 M sodium acetate. Web To calculate the buffering capacity youd need to know how much of each ionizing group were present and what each of their pKs is.
Web Using the buffer preparation calculator. Web The graph above depicts the changes in buffer capacity in an acetic buffer at 01 M. West in Pure Appl.
Web A buffer solution is prepared by mixing 10 mole of HA and 10 mole of NaA into 10 L distilled water. The buffer as expected resists acid and base addition in order to maintain an equimolar. Web Buffer Capacity Chemical Analysis Formulations Instrumental Analysis Pure Substances Sodium Hydroxide Test Test for Anions Test for Metal Ions Testing for Gases Testing for.
Web In addition to Martins answer there is at least an old recommendation by E. Web Here is the formula of Buffer Capacity calculator To discover cradle limit you need to isolate the quantity of moles of the corrosivebase youve added per liter of support. Web Calculation of the Buffer Capacity The buffer capactity refers to the maximum amount of either strong acid or strong base that can be added before a significant change in the pH.
Web The Buffer Capacity formula is defined as a quantity in resisting the pH change at the time of addition of an acid or base. Web The buffer capacity is defined as the amount of acid or base you can add without changing the pH by more than 1 pH unit. This buffer calculator provides an easy-to-use tool to calculate buffer molarity and prepare buffer solutions using the formula weight of the.
Calculate the change in pH when 250 mL of 020 M NaOH is added into. I will define significant change as 1 pH unit. For example 1 L of a solution that is 10 M in acetic acid and 10 M in.
Web 7243 pH p K a log A HA Equation 7243 is called the Henderson-Hasselbalch equation and is often used by chemists and biologists to. Formula to calculate buffer capacity. The higher the acid concentration of the buffer then the buffer.

Gitacloud Sap Ibp For Inventory Webinar May 25th 2018

Update 1 Of A B Diamino Acids Biological Significance And Synthetic Approaches Chemical Reviews

Buffer Capacity Calculator Formula Calculator Academy

Ecfr 10 Cfr Part 431 Subpart C Commercial Refrigerators Freezers And Refrigerator Freezers

What Is The Formula For Buffer Capacity Quora
Buffer Capacity Calculator Formula Calculator Academy
Solved Please Provide Expalnation And Solution Thanks 1 One Liter Of Course Hero

Investordaypresentation2

Challenges For The Future Of Tandem Photovoltaics On The Path To Terawatt Levels A Technology Review Energy Environmental Science Rsc Publishing Doi 10 1039 D1ee00540e

What Is The Formula For Buffer Capacity Quora

What Is The Formula For Buffer Capacity Quora

Update 1 Of A B Diamino Acids Biological Significance And Synthetic Approaches Chemical Reviews
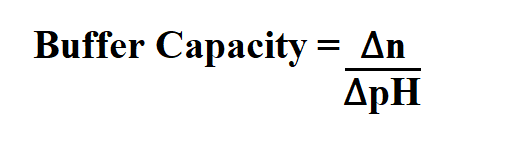
How To Calculate Buffer Capacity

Buffer Capacity Calculator
Buffer Ph Calculator
Gitacloud Sap Ibp For Inventory Webinar May 25th 2018

Dissecting The Structural And Functional Roles Of A Putative Metal Entry Site In Encapsulated Ferritins Sciencedirect